Equal Cell Division
Given the numerous components of a cell, how do animals keep any two cells similar? This fundamental question has received little attention compared to unequal or asymmetric cell division. Yet, equal cell division is deployed repeatedly by multicellular organisms. During early development, entire cell codes can be divided equally as evidenced by the presence of monozygotic twins. Later, after different tissues have been specified through unequal cell division, equal cell divisions can resume to generate similar cells that populate a tissue. Thus, organisms must also have mechanisms that control the switch between unequal and equal cell divisions.
We have begun to reveal mechanisms that ensure effective switching from unequal to equal cell division. We found that expression from repetitive DNA is particularly vulnerable to variation between cells. 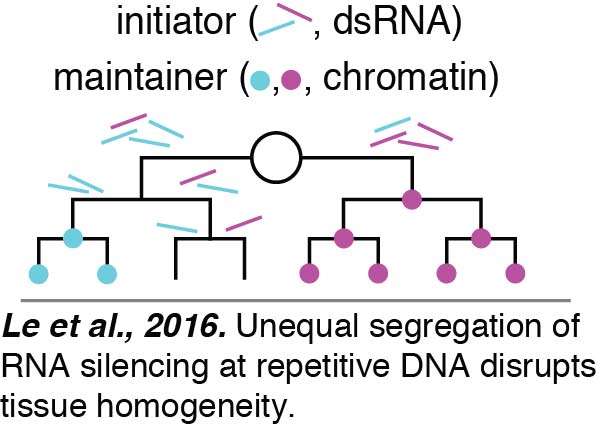 Our analyses suggests that this variation between cells results from unequal RNA silencing. An inhibitor of RNA silencing, ERI-1, that is inherited from parents as well as made in developing progeny acts in the embryo to eliminate such variation between cells and ensure uniform expression. In the absence of this factor, an initiator of gene silencing, likely double-stranded RNA (dsRNA), is segregated unequally between cells during the proliferative cell divisions required to make a tissue. This initiator causes threshold-dependent gene silencing. Consistent with the initiator being dsRNA, fewer cells show silencing when the dsRNA importer SID-1 is removed to block the spread of dsRNA between cells. Once initiated, the silencing is maintained by chromatin-mediated mechanisms despite DNA replication and cell division (Le et al., 2016).
Our analyses suggests that this variation between cells results from unequal RNA silencing. An inhibitor of RNA silencing, ERI-1, that is inherited from parents as well as made in developing progeny acts in the embryo to eliminate such variation between cells and ensure uniform expression. In the absence of this factor, an initiator of gene silencing, likely double-stranded RNA (dsRNA), is segregated unequally between cells during the proliferative cell divisions required to make a tissue. This initiator causes threshold-dependent gene silencing. Consistent with the initiator being dsRNA, fewer cells show silencing when the dsRNA importer SID-1 is removed to block the spread of dsRNA between cells. Once initiated, the silencing is maintained by chromatin-mediated mechanisms despite DNA replication and cell division (Le et al., 2016).
Once different tissues have been specified through unequal cell divisions and signaling in the early embryo, subsequent cell divisions within a tissue need to generate similar cells. Given the fundamental nature of this requirement, we suspect that many more processes in cells are under similar control to ensure a clean switch from differentiation to epigenetic memory during proliferative cell divisions that generate a tissue. Loss of such developmental mechanisms could potentially cause diseases later in life because of the generation of a variable population of cells within a tissue. For example, such pre-existing variation could increase the odds of age-related disease such as cancer that strike single cells apparently at random.
Back to research
Last updated: Feb 2018
Web Accessibility