Extracellular RNA
Cells can communicate with each other through the direct transport of macromolecules (e.g. RNA) in many organisms, including humans. Secreted vesicles with such macromolecules that are found in blood are being hotly pursued as valuable diagnostic markers for the health of internal organs. However, the mechanisms that enable this novel mode of cell communication are not well understood (see Jose, 2015 for review). The transport of RNA between cells had been inferred in C. elegans since the discovery of RNA interference. While such systemic silencing was apparent when dsRNA was fed to worms or injected into them, it was unclear whether dsRNA expressed in one cell can typically cause silencing in another cell. We established that many somatic cell types can export RNAs that enter other cell types and cause gene silencing (Jose et al., 2009) and identified a conserved tyrosine kinase as a regulator of dsRNA import into cells (Jose et al., 2012).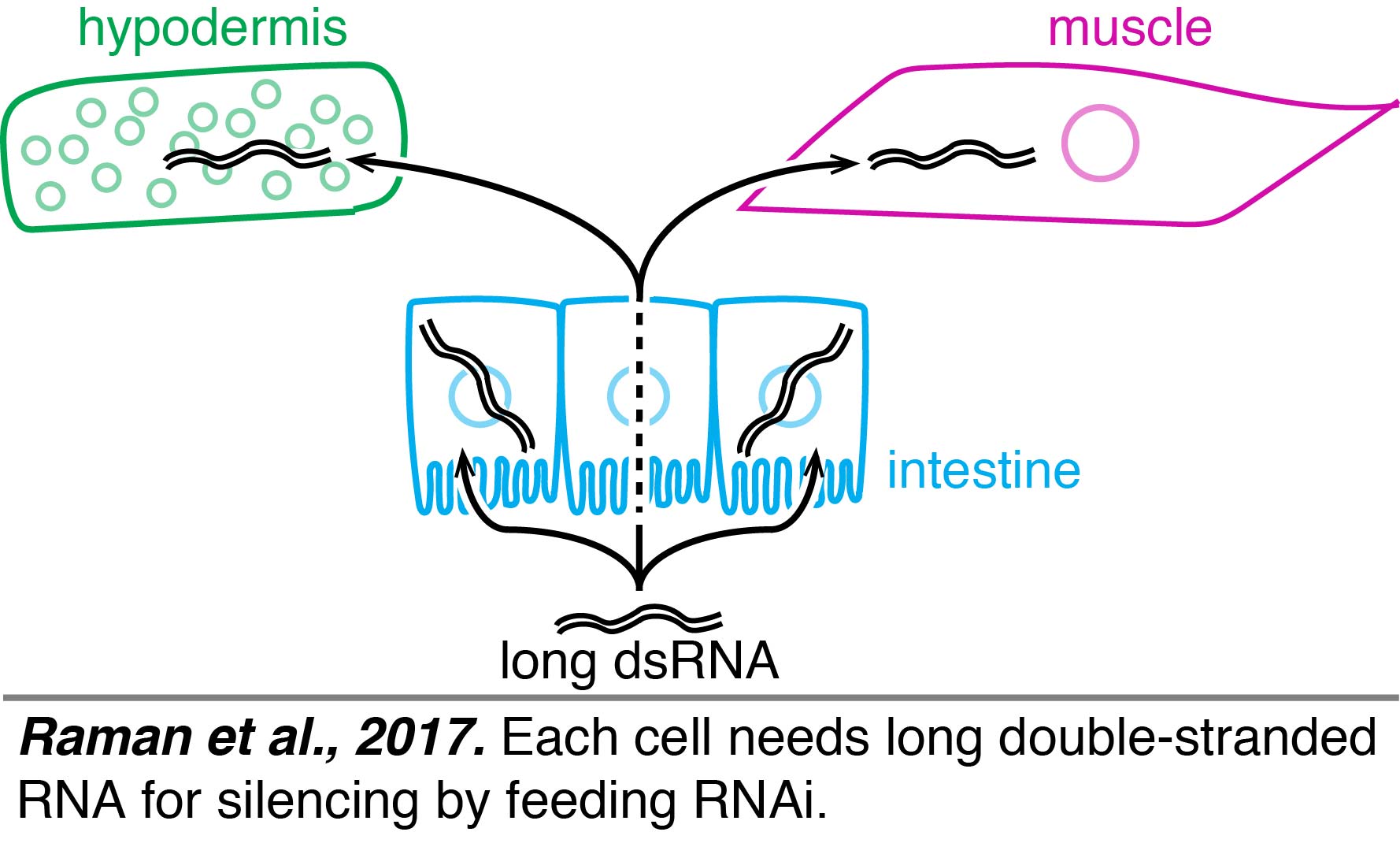 Tissue-specific rescue experiments had suggested that there were two forms of dsRNA that could move between cells – long dsRNA and short dsRNA – when long dsRNA was expressed from neurons (Jose et al., 2011). A similar conclusion was reached when ingested dsRNA was used to initiate silencing, suggesting that long dsRNA could enter a cell, be processed into short dsRNAs, and subsequently the short dsRNAs could move to other cells. This "transit" model for silencing by ingested dsRNA relied entirely on experiments that used repetitive transgenes for tissue-specific rescue, which can result in misexpression even when well-characterized promoters are used becasue of new promoters that can arise from DNA rearrangements (Le et al., 2016). Furthermore, expression from any repetitive transgene within a tissue can inhibit silencing of some genes by ingested dsRNA within that tissue (Raman et al., 2017), which could complicate interpretations of many past experiments that used feeding RNAi to study diverse problems in biology. A re-evaluation of the tissue-specific rescue experiments using single-copy transgenes showed that each cell needs long dsRNA for silencing by feeding RNAi (Raman et al., 2017).
Regardless of how dsRNAs are delivered into the worm – expression within a tissue, injection, ingestion, or soaking – dsRNAs are expected to reach the body cavity that surrounds all tissues in C. elegans. To directly visualize the fate of such extracellular dsRNAs, we injected fluorescently labeled 50-bp dsRNA into the body cavity. We found that dsRNA entered oocytes along with yolk and reached progeny (Marré et al., 2016). Surprisingly, we found that such delivery of extracellular RNA from parent to progeny did not require SID-1-mediated entry into the cytosol within the parent.
Tissue-specific rescue experiments had suggested that there were two forms of dsRNA that could move between cells – long dsRNA and short dsRNA – when long dsRNA was expressed from neurons (Jose et al., 2011). A similar conclusion was reached when ingested dsRNA was used to initiate silencing, suggesting that long dsRNA could enter a cell, be processed into short dsRNAs, and subsequently the short dsRNAs could move to other cells. This "transit" model for silencing by ingested dsRNA relied entirely on experiments that used repetitive transgenes for tissue-specific rescue, which can result in misexpression even when well-characterized promoters are used becasue of new promoters that can arise from DNA rearrangements (Le et al., 2016). Furthermore, expression from any repetitive transgene within a tissue can inhibit silencing of some genes by ingested dsRNA within that tissue (Raman et al., 2017), which could complicate interpretations of many past experiments that used feeding RNAi to study diverse problems in biology. A re-evaluation of the tissue-specific rescue experiments using single-copy transgenes showed that each cell needs long dsRNA for silencing by feeding RNAi (Raman et al., 2017).
Regardless of how dsRNAs are delivered into the worm – expression within a tissue, injection, ingestion, or soaking – dsRNAs are expected to reach the body cavity that surrounds all tissues in C. elegans. To directly visualize the fate of such extracellular dsRNAs, we injected fluorescently labeled 50-bp dsRNA into the body cavity. We found that dsRNA entered oocytes along with yolk and reached progeny (Marré et al., 2016). Surprisingly, we found that such delivery of extracellular RNA from parent to progeny did not require SID-1-mediated entry into the cytosol within the parent. 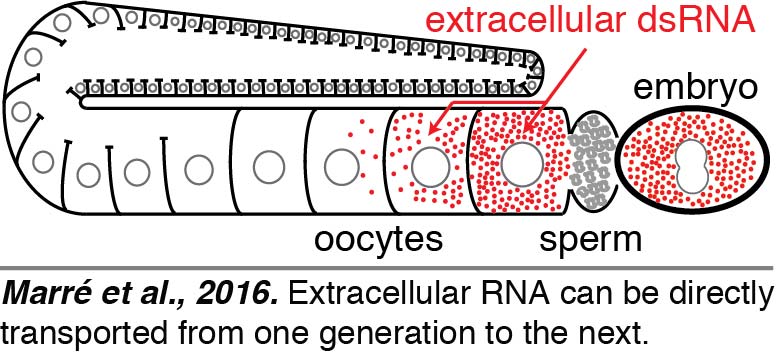 Thus, the dsRNA that enters oocytes along with yolk can presumably be held within intracellular vesicles and reach embryos. These results raise the intriguing possibility that extracellular RNAs that are secreted – potentially in response to changes in a parent – can directly reach progeny and regulate gene expression.
How dsRNA is exported from cells and the basis for differences in gene silencing by long and short dsRNAs are unknown. RNAs transcribed from the C. elegans genome or from ingested bacteria that accumulate extracellularly or that reach progeny are also unknown.
Thus, the dsRNA that enters oocytes along with yolk can presumably be held within intracellular vesicles and reach embryos. These results raise the intriguing possibility that extracellular RNAs that are secreted – potentially in response to changes in a parent – can directly reach progeny and regulate gene expression.
How dsRNA is exported from cells and the basis for differences in gene silencing by long and short dsRNAs are unknown. RNAs transcribed from the C. elegans genome or from ingested bacteria that accumulate extracellularly or that reach progeny are also unknown.
Back to research
Last updated: Aug 2017
Web Accessibility