|
› V-ATPase Overview
› V-ATPase 2002 Abstract
› V-ATPase 2002 Data
|
V-ATPase Trends Plant Sci 2002 Figures and Tables
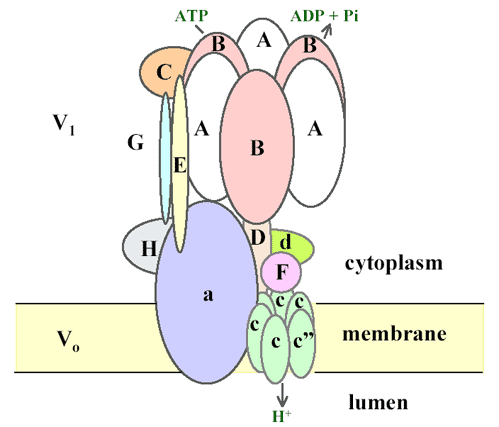
Figure 2. A revised model of the vacuolar-type ATPase adapted from Ref. [22]. The model is based mostly on topology studies of the vacuolar-ATPase (V-ATPase) from yeast and the bovine clathrincoated vesicle [21]. Electron micrographs of coated vesicle V-ATPase showed a peripheral stalk that runs from the top of the V1 to the Vo sector with space between the central and the peripheral stalk [23]. Subunit E is thought to form part of the peripheral stalk, linking the outer surface of V1 with Vo [22], rather than part of the central stalk [24]. Evidence suggests E is in contact with G, C and H. The cytosolic N-terminal domain of subunit 'a' is localized in the cytosol and associates with H and A [25]. The integral C-terminal domain of a subunit affects coupling of ATP hydrolysis to proton transport [26]. In yeast, each Vo complex contains all three types of c subunits [27]. Because subunit c2 is not found in the Arabidopsisgenome, we propose each Vo has one subunit c22 and five copies of subunit c. V-ATPase has been proposed to operate by a rotary mechanism similar to the F-ATPase [28]. In this model, ATP hydrolysis by the A3B3 hexamer held stationary by subunit a and the peripheral stalk drives rotation of the central core (D), which, in turn, causes the ring of c subunits to rotate. Subunit c carries the protons and the rotation of the c subunit ring is thought to be essential in driving proton transport.
Tables 1-3. These tables currently contain links to the PlantsT database (via the locus number), the MIPS database, and information on the TAIR website.
|

